Abortion pill case: Supreme Court rejects limits to mifepristone access
SCOTUS hears oral arguments in abortion pill case
Emma Waters with the Heritage Foundation joins LiveNOW's Andrew Craft with more insight after the Supreme Court heard oral arguments Tuesday over abortion pill access.
WASHINGTON - The U.S. Supreme Court has rejected a push to restrict access to the abortion pill mifepristone, a ruling that affects millions of American women.
In a unanimous decision, the justices ruled that abortion opponents lacked the legal right to sue over the federal Food and Drug Administration’s approval of mifepristone and the FDA's subsequent actions to ease access to it.
The decision is a loss for the group called the Alliance for Hippocratic Medicine, which sued the FDA over its approval of mifepristone, just months following the Supreme Court’s decision to overturn Roe v. Wade in 2022.
The group had asked the court to restrict access to mifepristone and to limit when in a pregnancy it could be used.
Abortion opponents initially won a sweeping ruling nearly a year ago from U.S. District Judge Matthew Kacsmaryk, a Trump nominee in Texas, which would have revoked the drug’s approval entirely. The 5th U.S. Circuit Court of Appeals left intact the FDA’s initial approval of mifepristone. But it would reverse changes regulators made in 2016 and 2021 that eased some conditions for administering the drug.
The Supreme Court put the appeals court’s modified ruling on hold, then agreed to hear the case, though Justices Samuel Alito, the author of the decision overturning Roe, and Clarence Thomas would have allowed some restrictions to take effect while the case proceeded.
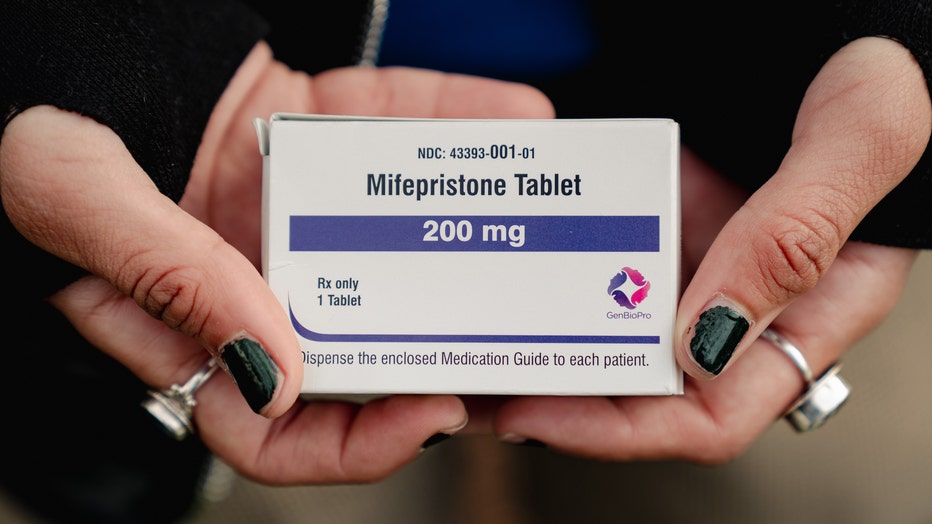
Box of mifepristone (Photo by Shuran Huang for The Washington Post via Getty Images)
The FDA approved mifepristone in 2000 as a safe and effective way to end early pregnancies.
Last year the pill was used in more than six in 10 of the abortions in the U.S., according to a recent study.
In 2016, FDA loosened restrictions on the drug to allow it to be prescribed up to 10 weeks of pregnancy and allowed nurses and other medical professionals to prescribe it. In 2021, the agency said the drug could be sent through the mail, doing away with a longstanding requirement that women pick the drug up in person.
OB-GYNs say a tiny fraction of patients suffer "major" or "serious" adverse events after taking mifepristone.
Since 2000, roughly 6 million patients have taken mifepristone, according to the FDA. A 2021 review of agency records looking for deaths that were likely related to the drug identified 13, or .00027% of patients.
This is a developing story. The Associated Press contributed to this report.

